
In the study of Favorskii mechanism using isotopic labelling the starting material was an equal mixture of 1(a) and 1 (b)
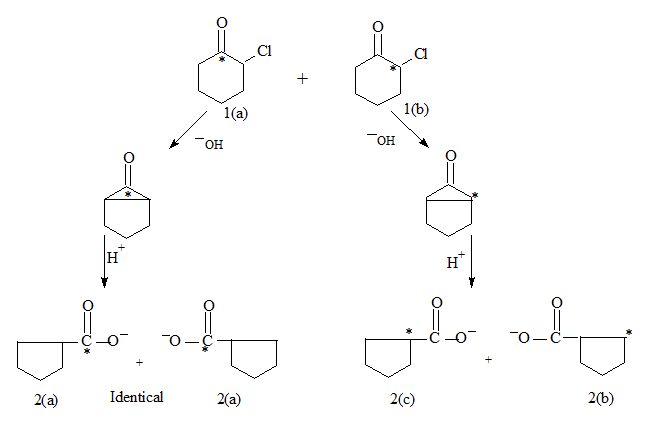
In basic medium the cyclohexanone derivative looses a proton and the resulting anion through an internal SN2 forms a cyclopropanone derivative as the intermediate.
The label is at the carbonyl carbon. This undergoes ring contraction in acidic medium resulting in the acid (or an ester).
The label now is at the α or β carbon 2(c) or 2(b) or at the caarbonyl carbon 2(a).
Thus the percentage of 14C
at the carbonyl carbon 2a is : 50%
at the α carbon 2(c) is 25% and
at the β carbon 2(b) is 25%
This is proved through the following degradation studies
Identification and estimation of the labelled carbon in the degradation products.
Each of the 14C from the compounds is got out as CO2 and is absorbed in Ba(OH)2 .The solid BaCO3 count is measured in a geiger account.
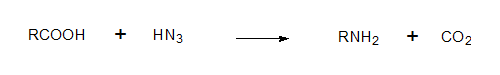
The carboxylic acids or reacted with HN3 (hydrazoic acid) converted to the corresponding amines (Schimdt reaction) and CO2 which is absorbed in Ba(OH)2. The BaCO3 count at this stage should be 50% of the starting material and this is what was found.
Note: In 2(a) the carboxylic acid contains the label hence degradation product is 14CO2
In the other carbxxylic acids 2(b) and 2(c) the label is in the ring system.
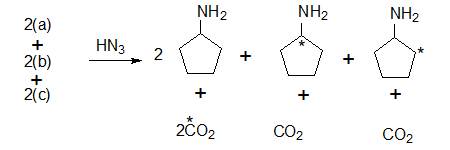
Further the amino cyclopentanes are oxidised to the corresponding cyclopentanones and hydrazoic acid would convert them to lactams (cyclic amides)
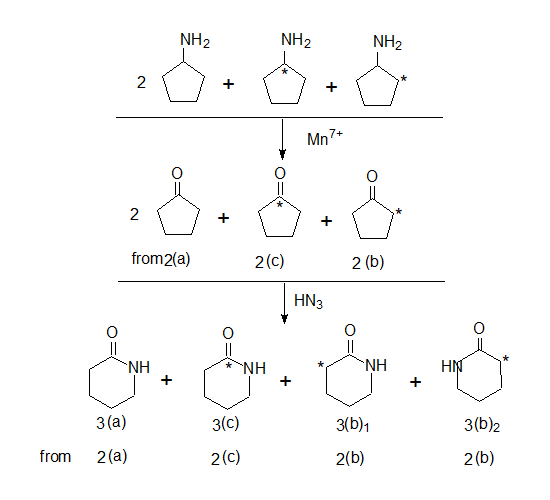
Note: When cyclopentanone 2(b) reacts with HN3 two cyclic amides are formed with one difference that is in the position of the 14C isotope 3(b)1 & 3(b)2.